MORPHOPTROTEOMICS
STUDY FOR A NEWLY-DIAGNOSED DIFFUSE LARGE B CELL LYMPHOMA CASE
Andy Nguyen,M.D./
UT-Medical School at Houston, Pathology/ Last Revision on: 8/30/2011
OBJECTIVES:
(1) Assignment of germinal-center–type versus non–germinal-center–type diffuse large B-cell lymphoma is performed with immunohistochemistry (Hans et al, 2004). This offers prognostic information for patients with newly-diagnosed diffuse large B cell lymphoma.
(2) If patient age is 50 year-old or more, EBER-1 will be performed. If EBER-1 is positive, the diagnosis is EBV-positive DLBCL in the elderly with poor prognosis (Swerdlow et al, 2008).
(3) For DLBCL cases in which the cell cycle analysis shows very high proliferative rate (measured as Ki-67 >80%), a more aggressive therapy known as R-Hyper CVAD is needed (Younes, 2004). A high proliferation rate (Ki-67) and a high mitotic index (more than 20 mitosis/10 high power fields) are indicative of a brisk cell cycle progression of the tumor cells from G2 into the mitotic (M) phase. Radiotherapy in the context of this brisk cell cycle progression of the tumor is likely very effective and can be considered as adjuvant to chemotherapy, especially for localized and bulky DLBCL (Connors, 2003).
(4)
For
DLBCL cases with increased NF-kappa B activity, proteosome inhibitors such as
Bortezomib may be considered in patients who fail R-CHOP.
They have also been proved beneficial to patients in initial treatment
(Leonard et al, 2007).
The resultant prognostic information, based on conventional chemotherapy (R-CHOP), may identify patients who could benefit from dose intensification (R-Hyper CVAD), radiation, or addition of a proteosome inhibitor. The principles of measuring activation status in signal transduction pathways are based on Morphoproteomics (Brown, 2008).
PROTOCOL:
For
a new diagnosis of DLBCL, the following 5 markers will be performed in the
diagnostic sample:
bcl-6
CD10
MUM-1
p-NF-kB (Ser 536)
Ki-67
Also
EBER-1 (
Results of study are to be reported in Addendum in order not to delay the diagnostic report.
BACKGROUND
Diffuse large
cell lymphoma is characterized by a high degree of chemosensitivity but
relapses frequently occur and many patients die from refractory disease
(Coiffier, 2005). The situation is worst for relapsing patients after
front line therapy without the option of allogeneic or autologous
transplantation. Therefore, new therapeutic strategies with adequate prognostic
data are clearly needed (Wanner et al, 2006).
Recently,
comparative gene expression experiments have shown that DLBCL can be divided into
at least three distinct subgroups: germinal center (GC)-like, activated B cell
(ABC)-like lymphomas, and primary mediastinal (thymic) large B cell lymphoma (Alizadeh
et al, 2000; Savage et al, 2003).
The assignment
of germinal-center–type versus non–germinal-center–type diffuse large B-cell
lymphoma seems to be able to be reproduced by studying the expression of
proteins using immunohistochemistry (Hans et al, 2004). The markers include bcl-6, CD10, and MUM-1. This set of markers has positive predictive
values of 87% and 73% for correctly identifying the germinal-center–type and
non–germinal-center–type, respectively.
This finding is particularly useful in light of the fact that morphology
alone is insufficient to differentiate these two types of DLBCL.
|
|
CD10 |
Bcl-6 |
MUM-1 |
|
GC |
+ |
|
|
|
GC |
- |
+ |
- |
|
ABC |
- |
+ |
+ |
|
ABC |
- |
- |
|
Primary
mediastinal large B-cell lymphoma (PMLBCL) represents less than 10% of all
large B-cell lymphomas, occurs primarily in young women, and always presents
with a mediastinal mass. The gene-expression profile
is similar to that seen in classical Hodgkin lymphoma (Savage, 2003). Currently,
there are no surrogate immunohistochemical stains that can be used to correlate
with the gene expression profile in PMLBCL. However, the diagnosis of PMLBCL can
be definitively established based on morphological features (large cells with
polymorphic nuclei that have an abundant rim of clear cytoplasm, fibrosis
resulting in compartmentalization of the neoplastic cells) and the
characteristic loss of surface immunoglobulins (Swerdlow, 2008).
Although the three subtypes of
diffuse large B-cell lymphoma do not have the same prognosis with
anthracycline-containing chemotherapy regimens (Rosenwald et al, 2002), they
are still treated in a similar way with CHOP regimen (cyclophosphomide,
hydroxydaunomycin, oncovin, and prednisone) [Fig. 1]. The addition of rituximab to CHOP has been a
major improvement in treatment of patients with diffuse large B-cell lymphoma
(Armitage, 2007). Rituximab has
neutralized the favorable prognostic value of bcl-6 and the adverse prognostic
value of bcl-2 known prior to the R-CHOP era.
Mechanism of therapy (JM
Connors, 2003):
-Cyclophosphomide (an
alkylator): induces covalent cross-linking of DNA strands and thus disrupts
nucleic acid metabolism, causing mostly cell death in S phase.
-Hydroxydaunomycin (an
anthracyclin, Adriamycin): complex formation with topoisomerase II and DNA,
leading to DNA strand break, causing apoptosis and disruption of DNA
transcription in G2 phase.
-Oncovin (a vinca alkaloid):
binds to tubulin, disrupting polymerization and mitotic spindle formation,
arresting metaphase cells in M phase.
-Prednisone: induces binding to
corticosteroid receptors on lymphocytes, leading to apoptosis and G1 arrest.
-Rituximab (CD20 antibody): (a)
induces complement-mediated cell lysis, (b) recruits antibody-dependent
cytotoxic T cells and NK cells, (c) induces cytotoxic memory cells, (d) induces
apoptosis directly, (e) down regulates bcl-2.
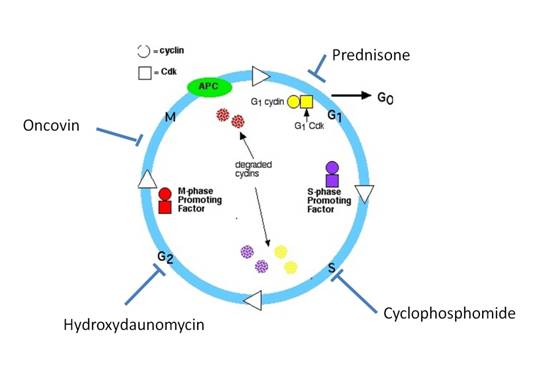
Fig. 1: Conventional CHOP regimen for DLBCL
For DLBCL cases in which the cell cycle analysis shows very high proliferative rate (measured as Ki-67 >80%), a more aggressive therapy known as R-Hyper CVAD is needed (Younes, 2004). This regimen consists of 4 cycles of R-CHOP, alternated with 4 cycles of Cytarabine (Ara C) and Methotrexate [Fig. 2].
Mechanism of therapy (JM
Connors, 2003):
-Cytosine
arabinoside (Ara-C): is an antimetabolic agent , which damages DNA when the cell cycle holds in
the S phase .
- Methotrexate competitively inhibits dihydrofolate reductase (DHFR), an enzyme that participates in the tetrahydrofolate
synthesis.
Folic acid is needed for the de novo
synthesis of the nucleoside
thymidine, required
for DNA synthesis.
Methotrexate, therefore, inhibits the synthesis of DNA in the S phase.
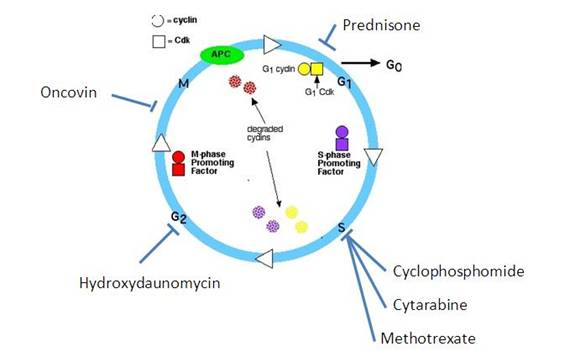
Fig. 2: Hyper CVAD regimen for DLBCL
A high proliferation rate (Ki-67) and a high mitotic index (number of mitosis/high power fields) are indicative of a brisk cell cycle progression of the tumor cells from G2 into the mitotic (M) phase. In general, cells are most radiosensitive in late M and G2 phases. The pattern of sensitivity correlates with the level of sulfhydryl compounds in the cell. Sulfhydryls are natural radioprotectors and tend to be at their lowest near mitosis. Radiotherapy in the context of this brisk cell cycle progression of the tumor is likely very effective and can be considered as adjuvant to chemotherapy, especially for localized and bulky DLBCL (Armitage, 2007).
The ABC group
is characterized by high expression of cyclin D2 and NF-kappa B activity [Fig. 3].
This gene signature is associated with a worse prognosis using conventional
chemotherapy. Proteosome inhibitors such as Bortezomib may be considered in
patients who fail R-CHOP regimen (Davis et al,
2001; Rosenwald et al, 2002). They have
also been proved beneficial to initial treatment (Leonard, 2007).
The NF-kB family comprises 5 members (p50, p52, p65, c-rel, and rel B) that form homo-dimers and hetero-dimers and function as transcription factors. They mediate proliferation, apoptosis, inflammatory and immune responses. NF-kB is important for normal B cell development and survival, and constitutively nuclear NF-κB has been implicated in a number of cancers including ABC type of DLBCL. In most cells, NF-kB is retained in an inactive form in the cytoplasm by binding to IkB proteins. In response to signaling through diverse pathways, IkB proteins are phosphorylated by the IkB kinase complex (IKK) and degraded by the ubiquitin-proteosome pathway. This leads to the release of NF-kB proteins that translocate into the nuclei and activate transcription (Fig. 3). Bortezomib (Velcade) is a proteasomal inhibitor that targets NF-kB by preventing degradation of its inhibitor, IκB.
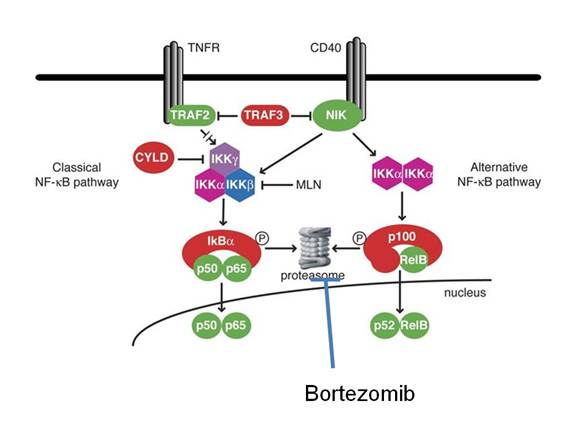
Fig. 3: NF-kappa B pathway
A partial illustration of signal transduction pathways (including mTOR, ERK, and NF-kB) is shown in Fig. 4.
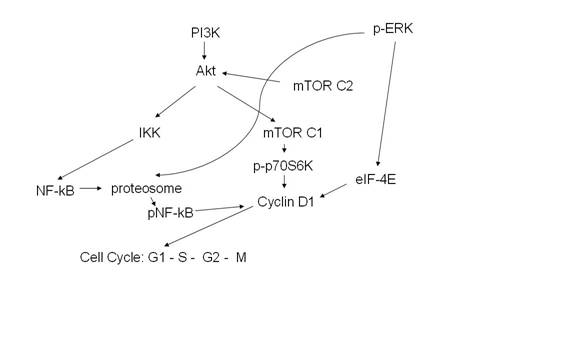
Fig. 4: Signal
transduction pathways
Legends: PI3K: phosphatidyl inositol 3-kinase, IKK: inhibitor
of KB kinase, pERK: extracellular signal regulated kinase, mTOR: mammalian
target of rapamycin, eIF-4E: eukaryotic translation initiation factor.
Similarly to ABC-group of DLBCL, PMLBCL is often found to have high expression of NF-kappa B activity. Proteosome inhibitors such as Bortezomib may also be considered in patients who fail R-CHOP regimen (Davis et al, 2001; Rosenwald et al, 2002). They have also been proved beneficial to initial treatment (Leonard, 2007).
In contrast, GC-like subtype of DLBCL is found to lack constitutive NF-kB activation (Wanner et al, 2006). This group is associated with better prognosis using conventional therapy.
If patient age is 50 year-old or more and EBER-1 is
positive, the diagnosis is EBV-positive DLBCL in the elderly with poor
prognosis. This would negate good
prognosis in GC-like subtype of D
REFERENCES
Brown RE. Morphogenomics and Morphoproteomics: A Role for Anatomic Pathology
in Personalized Medicine, Archives of Pathology and Laboratory Medicine: 2008, Vol. 133, No. 4, pp. 568–579.
Coiffier, B. (2005)
Treatment of diffuse large B-cell lymphoma. Current Hematology Reports, 4, 7–14
Wanner K, Hipp S, Oelsner M, Ringshausen I, Bogner C, Peschel C, Decker T. Mammalian target of rapamycin inhibition induces
cell cycle arrest in diffuse large B cell lymphoma (D
Alizadeh AA et al (2000). Distinct types of DLBCL identified by
gene expression profiling. Nature, 403:503-511
Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell
lymphoma differs from that of other diffuse large B-cell lymphomas and shares
features with classical Hodgkin lymphoma. Blood. 2003;102:3871-3879
Hans CP, Weisenburger DD, Greiner TC, et al.
Confirmation of the molecular classification of DLBCL by
immunohistochemistry using a tissue microarray. Blood.
2004;103:275-282
Rosenwald A, Wright G, Chan WC, et
al. The use of molecular profiling to
predict survival after chemotherapy for diffuse large-B-cell lymphoma. N
Engl J Med. 2002;346:1937-1947.
James O. Armitage. How I treat
DLBCL, Blood, 1 July 2007, Vol 110, no 1
JM Connors. Principles of Chemotherapy and combined
modality therapy. Chapter 12 in: Non-Hodgkin Lymphoma. Ed: PM Mauch et
al, Lippincott Williams & Wilkins, 2003
Davis, R.E., Brown, K.D.,
Siebenlist, U. & Staudt, L.M. (2001) Constitutive nuclear factor kappa B
activity is required for survival of activated B cell-like diffuse large B cell
lymphoma cells. Journal of Experimental Medicine, 194,1861–1874
Swerdlow, S. et al. World
Health Organization Classification of Tumours:
Pathology and Genetics, Tumours of Haematopoietic and Lymphoid
Tissue.
Leonard, J. P et al. CHOP-R plus bortezomib as initial therapy for
diffuse large B-cell lymphoma (DLBCL). Journal
of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol
25, No. 18S (June 20 Supplement), 2007: 8031
Younes, A. New treatment strategies for aggressive lymphoma. Semin Oncol. 2004 Dec;31(6
Suppl 15):10-3.
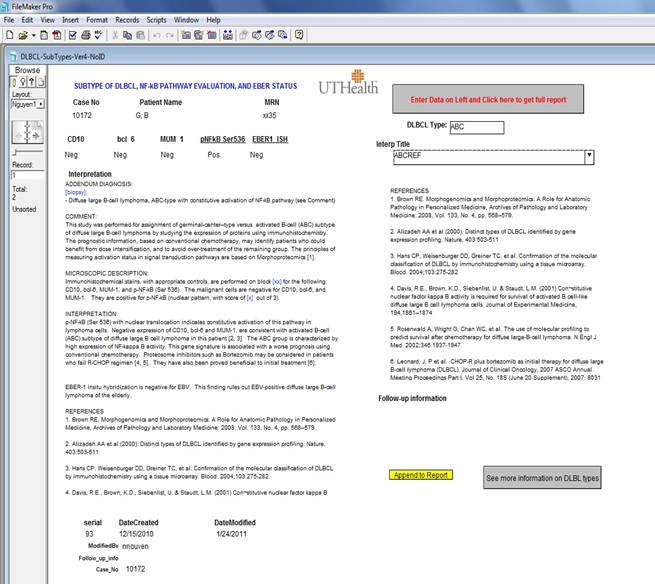
AUTOMATED REPORTING WITH FILE-MAKER PRO
Reporting morphoproteomics results
for DLBCL cases is done with FileMaker Pro.
The results of immunostains and ISH stain are entered in text boxes
together with accession number and patient’s identification, followed by
clicking the button “Enter Data on Left and Click here to get full
report”. A complete draft will appear on
the left panel that incorporates all the immunostain results (see Figure
below). The draft is based on the
algorithm outlined in “back ground”. This draft can be edited to obtain the
final report. The user can then
copy/paste this report into Cerner Pathnet (or APEasy) to release the final report.
The report in FileMaker Pro with accession number and patient’s identification can be
retained in the database for clinical follow-up in the future. An online version of this database has also
been made available on DPALM’s Filemaker Pro database server:
https://path.agillaire-h1.com/fmi/iwp/res/iwp_home.html